The Role of Ionization in Creating Quality Polymers

Ionization is probably not something you think of on a daily basis, but it is perhaps the most integral part of the process Gellner Industrial uses to create its high quality polymers. Gellner Industrial creates a specific type of polymers referred to as acrylic polymers. Acrylic polymers belong to a group of specific polymers that are known as plastics, essentially. They are durable, flexible and resistant to breakage. They have a number of different applications. Often they are used in the cosmetic industry as an integral part of nail polish. A lot of the acrylic polymers Gellner creates are used as paints, specifically, acrylic paints.
Whatever applications acrylic polymers are used in, they all have something in common. They all (except for non ionic acrylic polymers) regard a process called ionization and rely upon the use of ions. This is especially true in the case of anionic and cationic acrylic polymers. So what is ionization and what are polymers?
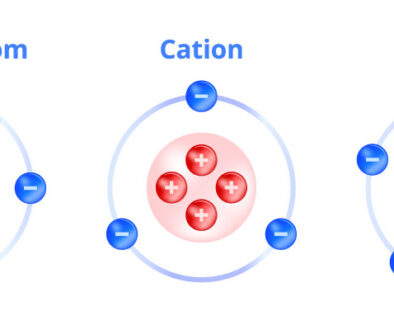
Ionization is a process in which atoms obtain an electrical charge. This electrical charge is obtained by the atom or molecule in some cases losing or gaining an electron to form an ion. An ion is this charged atom or molecule that has a charge because there is an inequity between the number of protons and the number of electrons in the molecule. An atom will have a negative charge if the number of electrons outweighs the numbers of protons inside the molecule. Similarly, an atom will have a positive charge if the number of protons outweighs the numbers of electrons inside the molecule. If the first case happens and the molecule has a negative charge, more electrons than protons, than the atom is a called an anion. On the other hand, if an atom has a positive charge, there are more protons than electrons, then this molecule is called a cation. Sound familiar? Gellner Industrial creates both cationic acrylic polymers and anionic acrylic polymers, meaning they create polymers with negative charges as well as polymers with positive charges. Examples of cations include: Silver: Ag+, hydronium: H3O+, and ammonium: NH4+ Examples of anions on the other hand include: hydroxide anion: OH–, oxide anion: O2-, and sulfate anion: SO42-
So now that you understand a little about the process of ionization and about ions themselves, as well as cations and anions, its important to know how these processes and terms come up. Ionization can with occur when there is a collision of an atom with other atoms, molecules, subatomic particles and ions, or through the interaction with light. Gellner Industrial creates ideal scientific conditions for these collisions and ionization as a whole to occur. It is through these arduous processes that Gellner can create such quality cationic and anionic polymers for all your business and personal needs.